According to its design and engineering, microfibers can be very different from each other.
Focusing on the most common cleaning textile microfiber, the bicomponent polyester (PET) -polyamide (PA, Nylon) pie wedge microfiber, here is short explanation of its physical properties:
+ Water absorption
Liquids are subject to the effect of surface tension which is the result of different intra molecular forces, and especially the Van der Waals forces. All liquids' molecules undergo cohesive forces between them which confers them this liquid physical state. However, molecules at the surface are subject to stronger cohesive forces than molecules down into the liquid since there are no other cohesive forces coming from above to compensate those cohesive forces coming from beneath the surface. Being bound to each other more strongly, surface molecules act as a film, making it more difficult to move an object through the surface than moving it when completely submersed. Water tension is what can make a needle float in a glass of water or what allows water striders to move on the surface of a pond.
Surface tension contributes to what is called the capillary effect, which takes effect when wicking away some liquid and can be observed when putting a dry sponge on a water puddle . Technically speaking, it occurs when the adhesive intermolecular forces between a liquid and another surface (in our previous example, respectively water and sponge) are stronger than the cohesive intermolecular forces of the liquid - In other words it is a mere power struggle! When adhering to the sponge surface, water molecules, because of their surface tension, stick together. Besides, because of the constant motion of liquid's molecules, new water molecules are consistently getting in touch with the sponge surface, progressing further on it... until the sponge is completely soaked. In some materials, such as a vertical glass tube, water is being pulled up until the weight of the lifted quantity, which causes a superior gravitational force, overcome the intermolecular forces of surface tension. The narrower the tube, the higher water can be drawn into it since a smaller mass of water is exposed to the surface allowing adhesive and cohesive intermolecular forces to combine and overcome gravity. Some materials which are water-repellent, such as Teflon, can prevent the capillary action. In that case the cohesive intermolecular forces of water are stronger than the adhesive forces with the Teflon surface, which prevent water from sticking to the surface .
To sum up, capillary effect's efficiency depends on the kind of the surface material and on the available surface in a given volume: the more the surface of a hydrophilic material available in a limited volume, the more efficient should be the capillary effect. Sponge remains the best example in this respect.
Why are microfibers absorbent?
Cleaning microfibers are engineered in such a way as to make them very sensitive to the capillary effect. The action of splitting microfibers is a clincher as it releases the polyamide star shaped core of the fiber, which is rather hydrophilic, while multiplying the number of strands available on a same volume. It thus proportionally increases the total added surface of all the fibers available and hence enhances the sorption properties of the microfiber fabric.
 |
|
 |
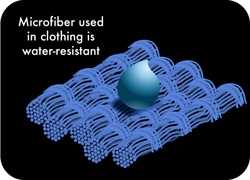
Weaving patterns are also important for thatpurpose as a very tightly woven polyester microfiber fabric will at first prevent water from soaking in since polyester is more water-repellent (which makes it efficient for clothing). Cleaning mops are thus woven with specific patterns that allow a high exposition of the fibers, especially the polyamide core, to the surface in order to maximize absorption .
The capillary action is also mechanically increased by the scrubbing movements during cleaning. On average, a can retain up to 8 times its weight in water. Once inside the microfibers, water will be distributed quite fairly between fibers since polyester, which generally constitutes most of the fibers, is rather hydrophobic and won't accumulate much water molecules on its surface. This property of PET microfibers prevent the fabric from soaking (unless completely submerged) and make it is easier to dry since water on the fibers' surface evaporates more quickly. Doing so, it also prevents the growth of bacteria inside the fabric. Generally,
moisture regain
of polyester is around 0,4%, polyamide around 4,5% and cotton around 7%.
When sweeping a wet surface, microfibers will absorb most of the liquid, but it will also spread some along the surface in a very thin layer, which, in case of water, evaporates almost immediately.
+ Dust trapping
Mechanical properties of microfibers
 |
|
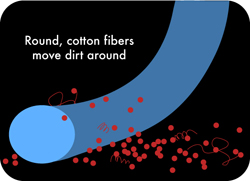 |
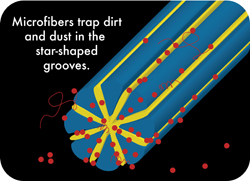
Once split, the cross section of regular cleaning microfibers will take different forms according to their composition. Polyester fibers will take the shape of wedges whereas nylon fibers will take the shape of stars with very thin branches (less than 1 micron in thickness). These angular forms have a much more efficient impact when cleaning a surface than regular round shaped fibers. The fibers' angles can lift the smallest dusts and even some bacteria. Besides the little space created between fibers after their splitting is narrow enough to trap all those particles . Each kind of fibers acts in fact as a microscopic tool with specific features that in an answer to specific needs
While using damp cleaning microfiber fabrics
Wetting a surface provokes an association of water and dust particles while wiping it with a cleaning microfiber mops achieves to absorb the liquid and trap the dusts inside the fibers. There again, surface tension of water is involved in retaining the dust particles and make them stick to the fibers' surface. Those particles are, in turn, trapped between the strands of the microfiber.
While using dry cleaning microfiber fabrics
Static electricity is one of the first electrical effects to have been studied. Ancient Greeks discovered that rubbing some materials against each other could make them attractive to small particles. This effect is explained by an imbalance thus created in the amounts of positive and negative charges found within the surface of an object. Static electricity occurs when two object with an opposed charge are brought close to each other so as to create an electric field. This electric field is responsible to many properties and can be compared, to some extent, to a magnetic field. The bigger the gap between the two opposite charges, the more static electricity. This gap is measured in volts and often involves high voltage. When created by human activity, static electricity can range up to several thousands of volts. Everyday life has a broad range of electrostatic manifestations, from the small discharge one can get while gripping a doorknob to the phenomenon of lighting during a storm.
Static electricity accumulated while putting two insulated surfaces into contact is known as triboelectric charging and depends on different parameters. The more surfaces are put into contact, the more they accumulate a charge. In this respect rubbing allows a repeated contact with few movements and further increases the voltage. According to the materials, the charge accumulated will be either positive or negative, whereas other materials do not accumulate charges at all. Triboelectric series list materials according to their charging polarity and capacity:
|
POSITIVE CHARGE |
NEUTRAL |
NEGATIVE CHARGE |
||
|
|
Dry Human skin |
Cotton |
|
Wood |
The ability for a material to acquire a positive or a negative charge tallies with its ability to lose or gain electrons. Concretely speaking, when putting two surfaces into contact, some chemical bonds are created and when surfaces are separated, bonds rupture tends to leave imbalanced charge behind, as electrons will move from one surface to another according to the material involved. The surface gaining electrons will acquire a negative charge whereas the surface losing electron will acquire a positive charge. Being insulated from the rest of its environment, generally by the air, the charged object can recover its neutrality if brought close enough to something with an excess opposite charge or to a neutral conductor. The charge can also dissipate after time especially if there is some humidity in the surrounding air. Incidentally, when there is a high degree of humidity in the atmosphere, electrostatic phenomenon are much more seldom since the water particles in the air act as a conductor and neutralize the excess charges on the surfaces.
What's in it for electrostatic microfiber?
Static electricity is the key behind this somehow magical effect: when fully efficient, a charged microfiber cloth do not even need to make contact with dust, it just attracts them from where they lie.
Microfibers are made of polymers that have interesting triboelectric properties: polyester and nylon. When using a microfiber mop to sweep the floor, the fibers are rubbed between them and with the soil surface. Polyester fibers acquire a positive charge while nylon fibers acquire a negative charge, creating an efficient electric field. The charged mop will then attract small particles of dust through electrostatic induction, a by-effect that modify the charge within the dust particles.
Split microfibers have very interesting electrostatic properties since nylon fibers are released amongst polyester fibers and their respective surfaces are in direct contact. Moreover the total added surface of all the fibers available being considerably increased, weeping movements will consecutively enhance the contact between fibers and the floor, and thus the electrostatic potential.
Note that wet microfiber will lose its static electricity efficiency since water is rather conductive and when spread on the surface of the fiber it prevents the concentration of electric charges. In this regard, the low moisture regain of polyester is a favorable to its electrostatic properties.
Grease/fat absorption
The molecular composition of polyester and nylon make them highly lipophilic . It means that fatty substances adhere to their surfaces. In fact both polyester and nylon are originally made of oil products! Once again split microfibers while offering a wider available surface can scoop up fatty substances much more efficiently than other fibers. Fat stay on the surface of the fibers until the cloth is laundered.
Microfiber, a clever fiber
The regular bicomponent pie wedge cleaning microfibers, with their specific structures and properties, are precision tools and as such a lot of care has to be taken during their manufacture. However nothing looks like a cloth more than a cloth and it is impossible to distinguish between microfibers with a naked eye. One can only perceive the difference while in use.
As stated before microfibers offer a vast array of uses and can adapt many specific needs. Cleaning microfibers are not restricted to the regular bicomponent pie wedge . Different patterns exist and new ones make their appearance every now and then. However their properties are similar to those described. As for apparels, microfibers have been long employed for their water repelling and breathing properties. But many more applications have appeared since then involving advanced technologies: anti-bacterial and anti-odour properties, electricity generation.
All of this contributes to create the next generation of textile, known as smart fabric.






 n
n